昆明,中国,2017年10月15日
CONTROL ID:2776800
PRESENTATION TYPE:Poster Only
CURRENT CATEGORY:Steatosis and Steatohepatitis
CURRENT DESCRIPTORS:SO1. Steatohepatitis: Experimental
TITLE:Development and Characterization of High Fat Diet-induced Non-human Primate Models of Liver Diseases:Steatosis, NASH and Fibrosis
AUTHORS (FIRST NAME, LAST NAME):Lichuan Yang1, Joseph Gabriel1, Zhenghua Zhu1, Feng Li1, Yuchao Zhao1, Guanzhong Wang1, Rosario Perez1, Shaodong Li1, Tony Wang1, Bob Zhang1
Oral/Poster: Institutional Author(s):(none)
INSTITUTIONS (ALL):
1. Kunming Biomed International, Kunming, Yunnan, China.
ABSTRACT BODY:
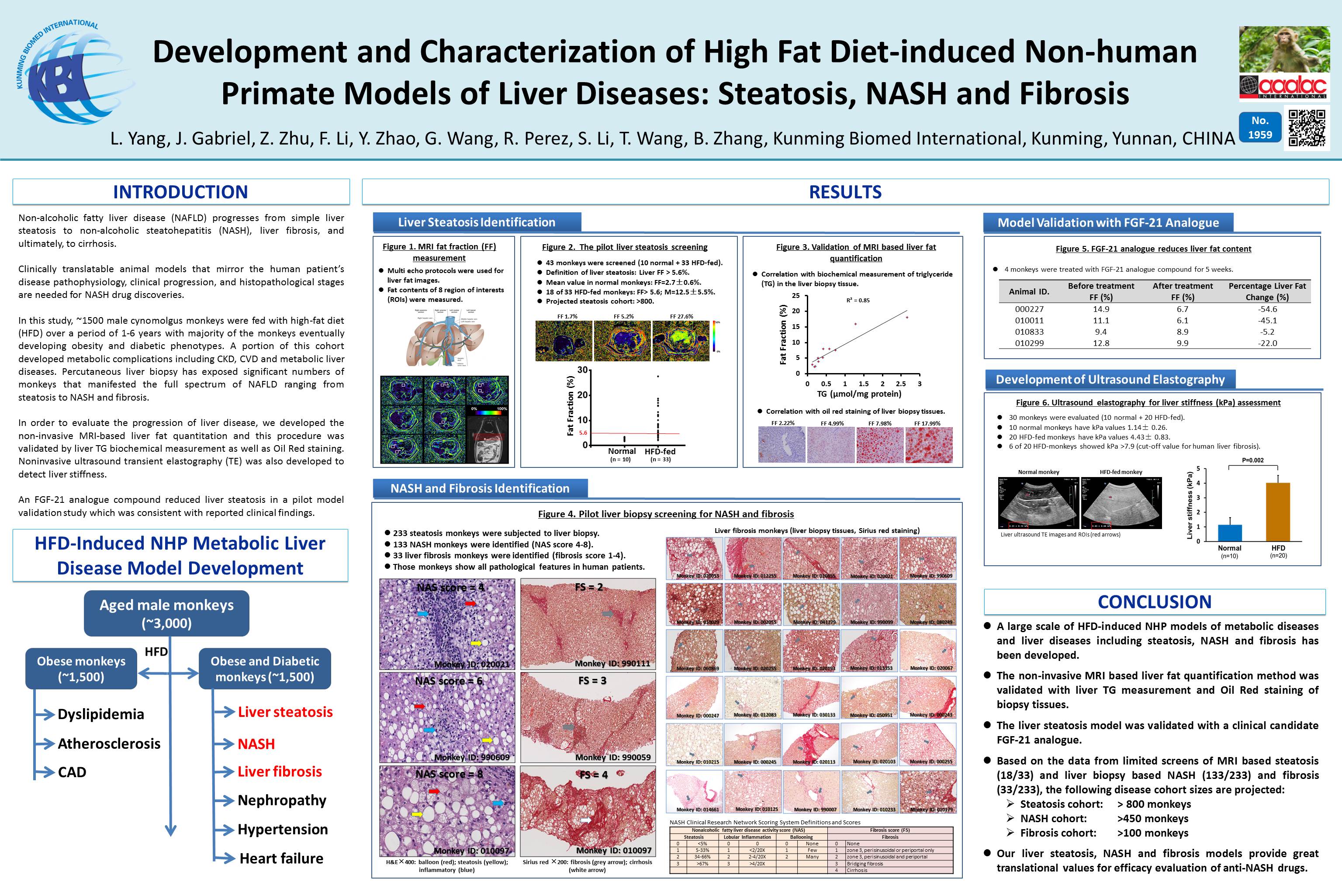
Abstract Body:Background: Lack of translatable animal models is a major challenge for developing nonalcoholic steatohepatitis (NASH) therapies. This study aims to develop a large colony of high fat diet (HFD)-induced nonhuman primate (NHP) liver disease models to facilitate new NASH drug discovery.
Methods: Over 1,500 male cynolmolgus monkeys were fed with HFD over 2-6 years. Metabolic syndrome phenotypes and diabetes progression were monitored longitudinally by body weight, IVGTT, BP and clinical chemistry. Liver steatosis was measured by ultrasound liver scan and quantitated by MRI. MRI assessment was validated by liver biopsy and hepatic triglyceride (TG). NASH was assessed by histological analysis of liver biopsy after HE staining. Liver fibrosis was evaluated by ultrasound elastography for stiffness (kPa) and biopsy after Sirius Red staining. NAFLD activity score (NAS) and fibrosis score (FS) were generated by the scale from NASH Clinical Research
Network.
Results: Majority of HFD-fed monkey colony developed metabolic syndrome phenotypes, as well as diabetes and diabetic complications. MRI liver fat fraction (FF) measurements performed in a representative cohort of 33 HFD-fed monkeys showed that 18 monkeys (55%) had FF >5% (cutoff value for steatosis), with average of 12.4±5.5%; while 10 normal monkeys had FF value of 2.7±0.6%. MRI FF levels correlated well with Oil Red O and HE staining of liver biopsies and hepatic TG levels (R2=0.85, p<0.05; n=12). Limited liver biopsy on monkeys (N=62) demonstrated 40 monkeys (64%) had NASH phenotype (NAS score of 4-7), including severe steatosis, moderate ballooning, and lobular inflammation. 20 of these NASH monkeys (32%) showed perisinusoidal/portal/periportal/bridge fibrosis (FS scores 1-3). Ultrasound elastography showed higher liver stiffness in NASH monkeys (kPa=4.79±1.09, n=10) compared to normal monkeys (kPa=1.14±0.25, n=10; p<0.009). 4 of 10 NASH monkeys had kPa >7.7 (cutoff value for human fibrosis). Their fibrosis stages were confirmed by liver biopsy.
Conclusion: HFD-induced NHP liver disease models have been developed and characterized in a large cohort. They have similar disease progression and pathological features seen in humans. Based on limited MRI and liver biopsy data, it is estimated that 55% of monkeys on HFD diet have steatosis (>800), 64% of steatosis monkeys have NASH (>500) and 32% of NASH monkeys have fibrosis (>150). These models have potential translational values for therapeutic evaluation of anti-NASH agents.
(no table selected)
(No Image Selected)
Co-Author Disclosure Status
The following authors have completed their AASLD 2017 disclosure:Lichuan Yang: Disclosure completed | Joseph Gabriel: Disclosure completed | Zhenghua Zhu: Disclosure completed | Feng Li: Disclosure completed | Yuchao Zhao: Disclosure completed | Guanzhong Wang: Disclosure completed | Rosario Perez: Disclosure completed | Shaodong Li: Disclosure completed | Tony Wang: Disclosure completed | Bob Zhang: Disclosure completed
AASLD 2017 poster 2017-10-05.jpg
美凤力借多年的大动物经验,坚持以“务实求真”为宗旨,累计为4000多家客户提供品质大动物临床前服务,得到了客户的一致好评。如果您有动物试验、临床培训、组织病理、大动物试验、临床试验、产品注册科研课题等...
请立即点击咨询我们或拨打咨询热线: 17312606166 ,我们会详细为你一一解答你心中的疑难。 添加好友




 17312606166
17312606166